Why EHDS will change the rules of healthcare in the EU
The goal of EHDS is to unlock the potential of health data – securely, responsibly, and across borders.

For years, fragmented systems across member states have stood in the way of better patient care and slowed down innovation.
The European Health Data Space (EHDS), one of the most ambitious digital health projects the EU has ever taken on, is changing that.
This is the EU’s first sector-specific data space. That alone says a lot. It’s not just about storing health data. It’s about creating a shared framework that balances two priorities: enabling better healthcare and protecting individual privacy and security at the highest level.
Why was EHDS created?
Despite the rise of digital tools, healthcare systems across Europe are still disconnected. That’s left patients struggling to access their medical records when they travel or move within the EU. It’s also made it harder for doctors to make well-informed decisions.
EHDS addresses this with what’s called the primary use of health data, ensuring that information like patient summaries and e-prescriptions can move with people across borders.
But it doesn’t stop there. EHDS also supports the secondary use of health data, such as research, innovation, education, policy-making, and more.
Today, health data is often siloed, making it hard for researchers, regulators, and developers to get the big-picture insights they need to make meaningful progress. EHDS creates a framework to responsibly open up that data, without compromising privacy.
Beyond the technical hurdles, EHDS is also about meeting growing expectations. Patients want easy, secure access to their own health data – and, this is something today’s systems can’t always deliver. And they want that level of care no matter where they live. EHDS aims to reduce the digital divide across member states and bring more consistent, high-quality care to everyone.
Who’s involved in EHDS?
To bring this system to life, EHDS introduces several key roles:
- health data holders – organisations like hospitals, research institutions, healthcare providers, and even app developers who generate or manage health data. They’ll need to catalogue and regularly update metadata about their datasets and share them, either directly or through national intermediaries, for approved secondary uses.
- health data users – individuals or organisations (researchers, public institutions, or educational bodies) who get approved to access health data for purposes like medical research, innovation, or statistical analysis.
- health data access bodies (HDABs) – every EU member state will establish a national HDAB to oversee access to health data. These entities process access requests, ensure data is used responsibly, and make sure the system runs smoothly and securely.
- trusted data holders – some organisations that meet strict security and technical requirements may be given a special status. These entities can provide direct access to health data through secure environments, which helps streamline processes while still maintaining compliance and control.
All entities handling electronic health data are expected to comply with EHDS regulations, unless they’re microenterprises (fewer than 10 employees and turnover under €2M), and even that exemption can be lifted by national authorities.
The roadmap: What happens when?
EHDS isn’t going live all at once. It’s being rolled out gradually to give stakeholders time to prepare and adapt.
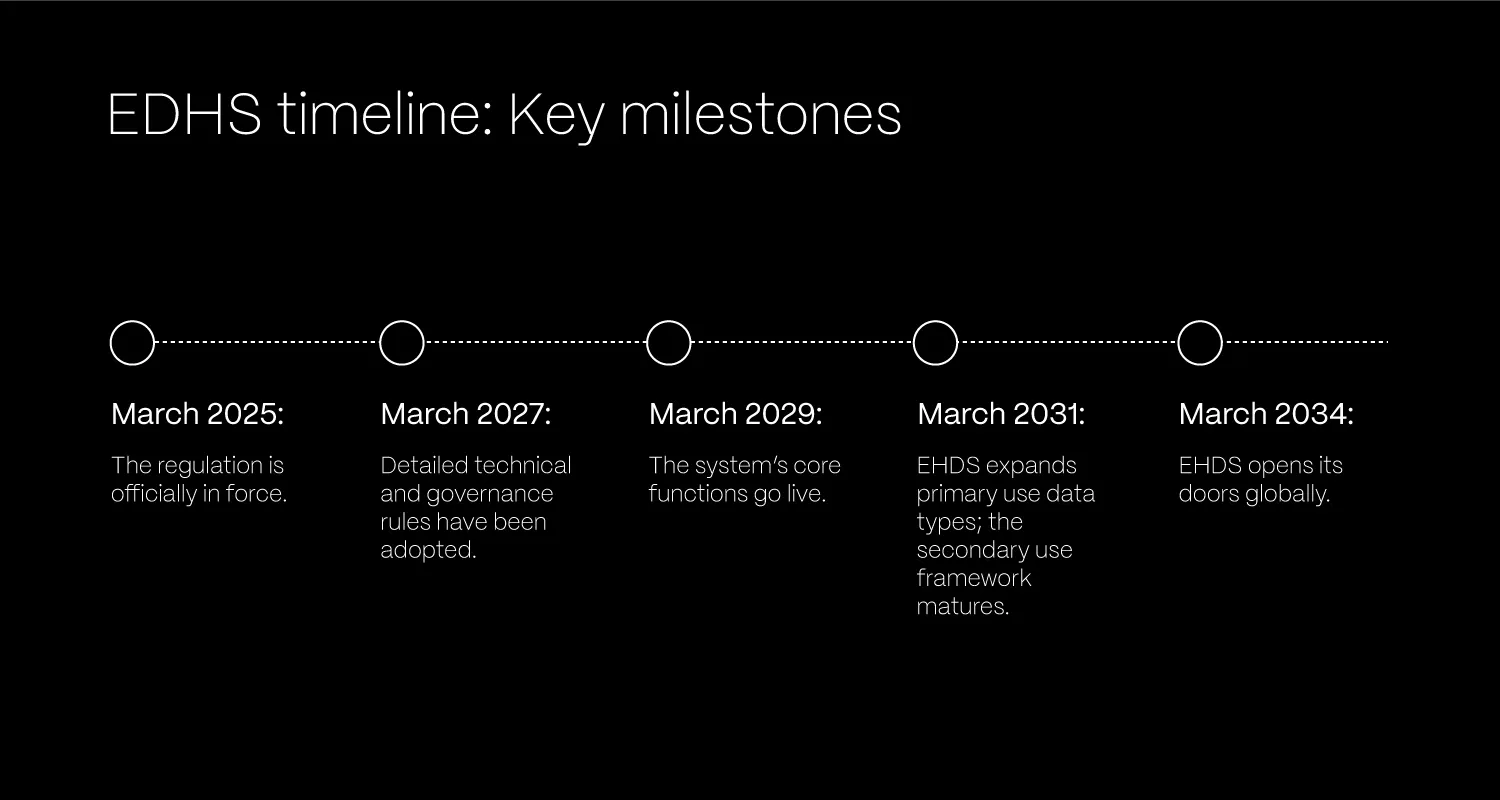
- March 2025: The regulation is officially in force.
- March 2027: Detailed technical and governance rules (implementing acts) will be adopted.
- March 2029: The system’s core functions go live. That includes the exchange of essential health data, like patient summaries and e-prescriptions, across borders. The secondary use of general health data also begins.
- March 2031: EHDS expands to support more data types (like medical images, lab results, and hospital discharge reports) for primary use. The secondary use framework also matures to include all data types, including genomic data.
- March 2034: EHDS opens its doors globally. Third countries and international organisations can apply to use the HealthData@EU platform for secondary purposes, strengthening global health research collaboration.
Where do we start?
You don’t need to wait for 2027 to take action. Many of the regulation’s foundations are already clear, and there’s plenty of actions organisations can do now to get ahead and avoid a last-minute scramble.
Define your role
Start by identifying whether your organisation is a data holder, a data user, or both. This will shape your responsibilities under EHDS, from what data you’ll need to provide, to how you manage access, compliance, and interoperability.
Map your health data
Run a full audit of the health data in your systems. You’ll need to identify:
- the types of data (EHRs, genomics, administrative, etc.);
- where the data comes from (internal, external, third-party);
- legal bases and current processing activities;
- conditions for sharing;
- technical structures and metadata.
Think of this process not just housekeeping. It’s a regulatory must. National Health Data Access Bodies will use this metadata to assess access requests. Getting it right matters.
Align your tech stack
EHDS sets a high bar for interoperability. Systems will need to support the European Electronic Health Record Exchange Format (EEHRF), meaning alignment with standards like HL7 FHIR or openEHR is critical.
If you’re not there yet, it’s time to assess the gaps and build a roadmap toward compliance. Your systems should also be ready to:
- generate standardised, machine-readable metadata;
- support advanced logging, auditability, and access controls;
- enable secure processing environments.
Wrapping up
The European Health Data Space opens the door to smarter services, faster research, and more personalised patient care – all built on trusted, interoperable data.
Organisations that start preparing today won’t just stay ahead of regulation. They’ll position themselves as leaders in a health ecosystem that’s finally catching up with the digital age.