Introducing Axceler8 RX, an innovative clinical trial management solution
Bringing life-changing treatments faster

We’ve created an innovative clinical trial acceleration platform that helps pharmaceutical companies bring life-changing treatments to patients faster.
Built on deep domain expertise and advanced technical capabilities, it streamlines a critical segment of the trial process such as removing bottlenecks, optimising workflows, and providing the visibility needed to make smarter decisions. Because when clinical trials run faster and smoother, everyone benefits, especially the people waiting for new therapies.
Addressing the complexity and challenges of clinical trial management
Clinical trials are long, costly, and operationally complex. They span multiple years, involve teams across continents, and require precise coordination among clinical centres, hospitals, and research organisations.
Patient recruitment remains the biggest challenge:
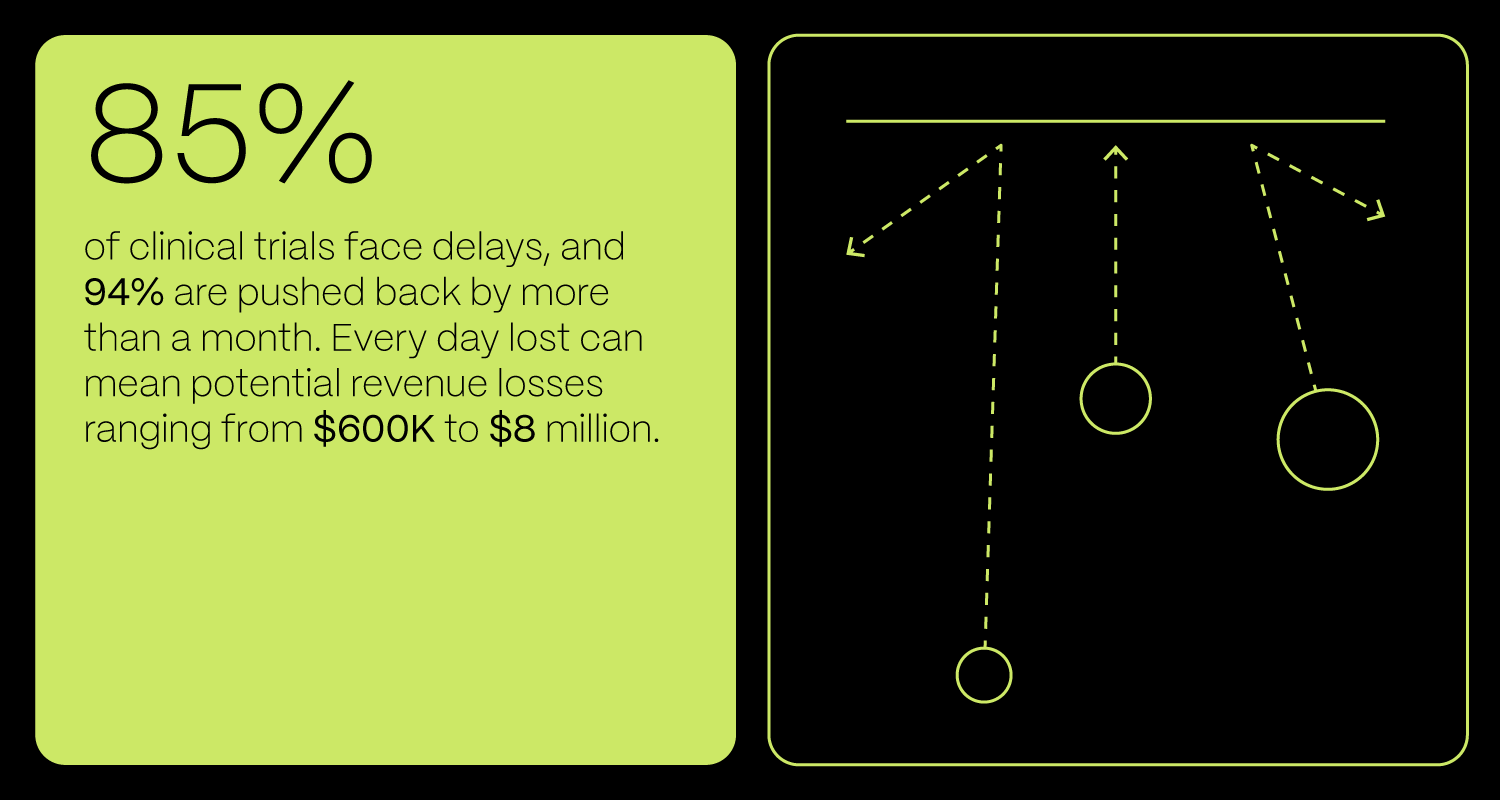
- 85% of clinical trials face delays
- 94% are delayed by more than a month
- Delays can cost $600K to $8M per day, with nearly half caused by recruitment and retention issues
Added to this are difficulties in tracking progress, accessing real-time data, and applying advanced analytics to guide decisions. All of this slows trial execution, and delays treatments reaching patients.
To address these challenges, we created a scalable, future-ready platform that streamlines trial management, improves efficiency, and reduces operational costs.
Introducing Axceler8 Rx – a breakthrough in clinical trial management
We joined forces with Arthur D. Little, the world’s first management consulting firm, with expertise in innovation, technology, and strategy, and created Axceler8 Rx. The product is a modern, single-tenant platform designed specifically for pharmaceutical companies. Its main goal is to enhance efficiency, streamline operations, and support large-scale trial management.
The platform is built around two key capabilities: clinical trial modelling and monitoring.
Clinical trial modelling
This is a forecasting process that happens before a clinical trial starts. The idea is to use as much relevant information as possible to predict how the trial will unfold.
In this case, the platform pulls in diverse industry data sources such as historical trial results, patient demographics, disease prevalence, regulatory timelines, and operational metrics. It then applies domain expertise to process this data.
As part of this modelling, Axceler8 Rx also helps sponsors answer two core questions early: where to run the trial and how enrolment will behave over time.
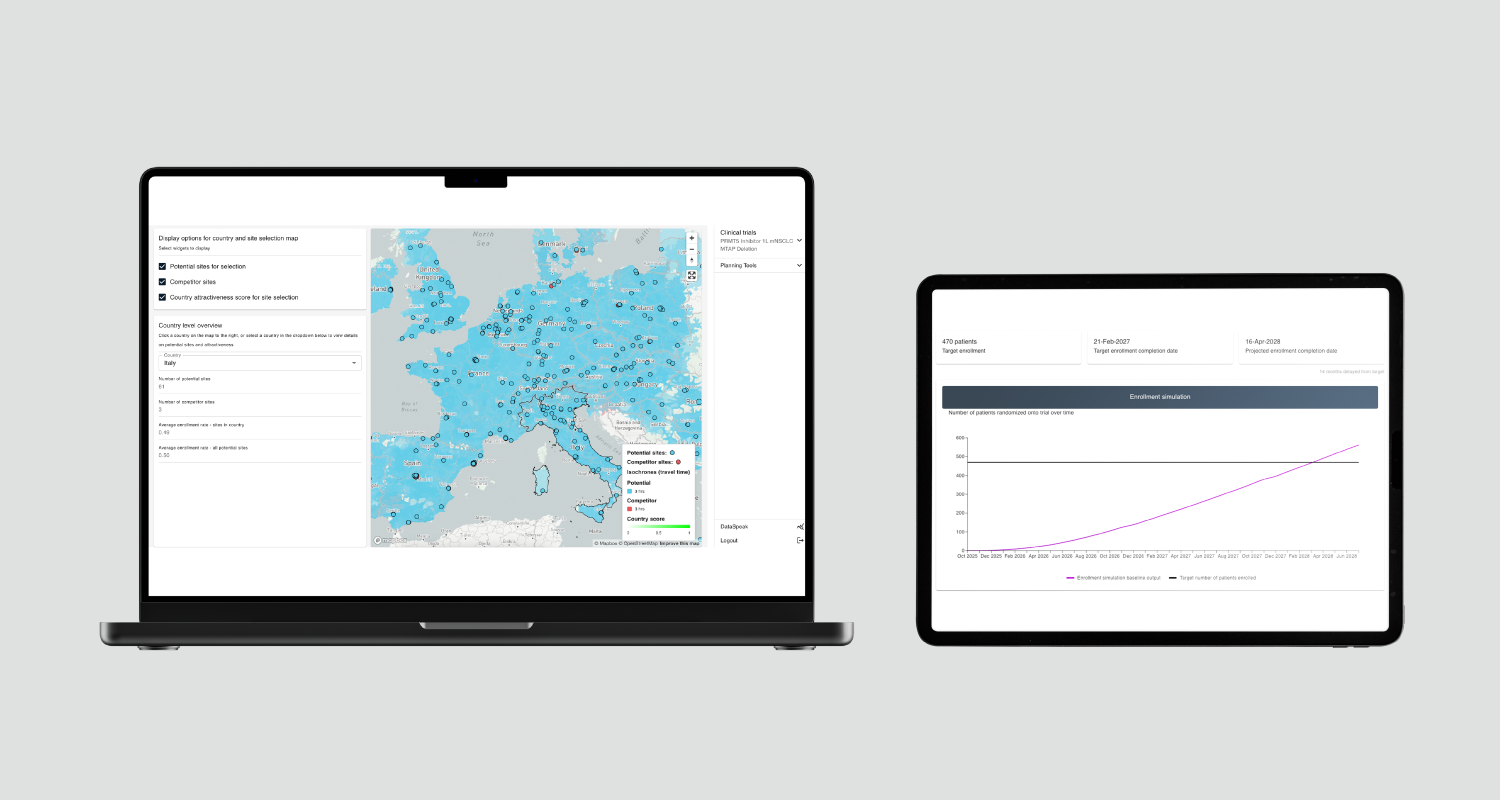
- The AI Site Selection module identifies the most suitable countries and sites based on historical performance, feasibility inputs, and capacity, helping teams design a realistic and deliverable site mix.
- The Enrolment Forecast module uses Monte Carlo simulations to model thousands of scenarios and generate expected enrolment curves before the first patient is enrolled.
- Teams can also run “what-if” scenarios, adjusting the number of sites, eligibility criteria, or geographies, to reveal design flaws early and plan resources more accurately.
By combining data, simulation and scenario analysis, the platform predicts:
- Trial progression – how the trial is likely to advance step-by-step, including milestones and possible bottlenecks.
- Outcomes – likely success rates, potential issues, or risks to address in advance.
- Timelines – realistic estimates of how long the trial will take from recruitment to completion.
This modelling helps pharmaceutical companies plan better, allocate resources efficiently, and avoid costly delays before the trial even starts.
Clinical trial monitoring
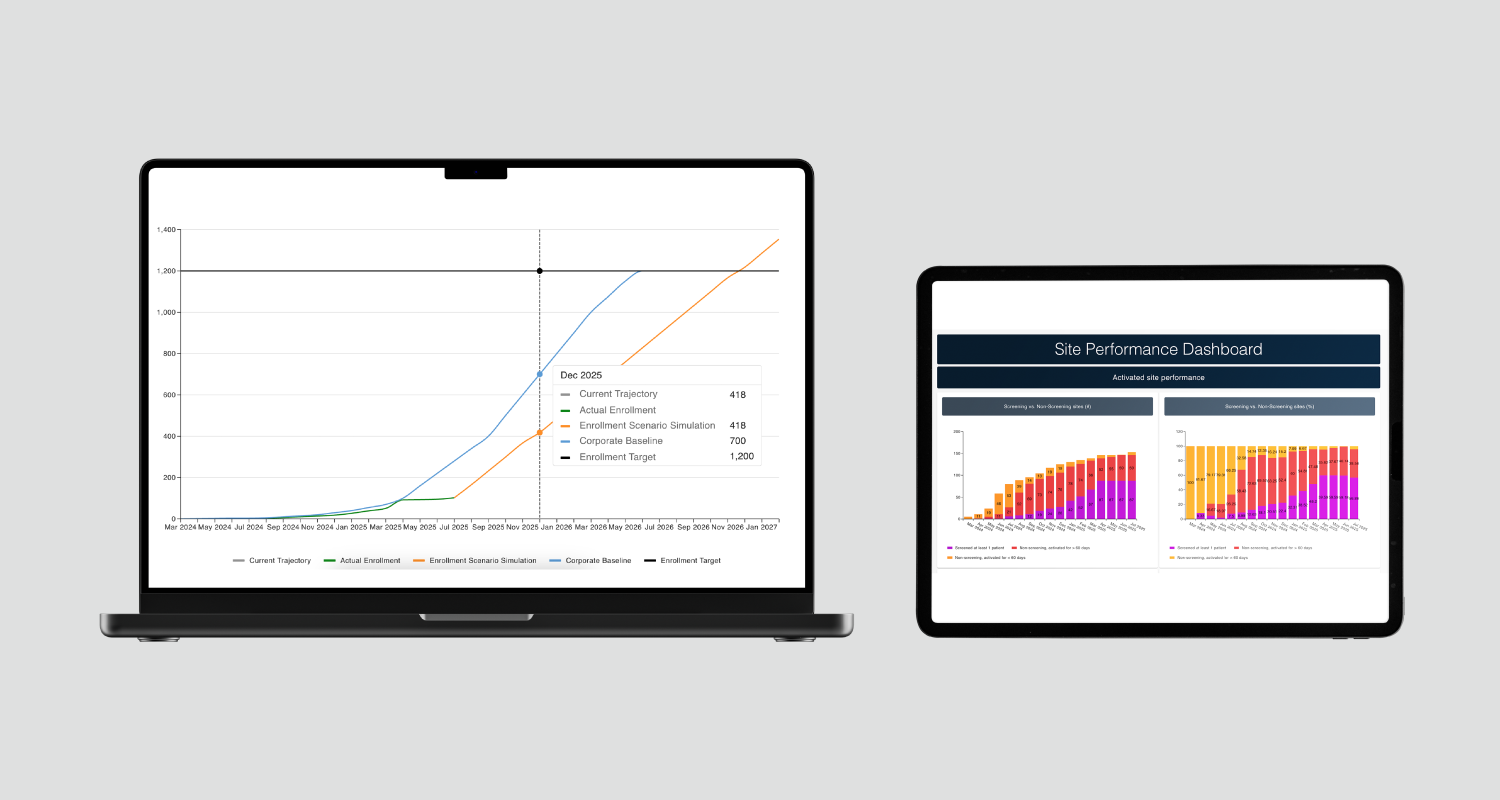
Monitoring provides continuous insight into how a trial is progressing across countries and sites. Axceler8 Rx tracks enrolment progress, site performance, data completeness, and compliance milestones, providing study teams with a clear real-time understanding of trial status.
- The Recruitment Tool gives up-to-date visibility into enrolment and data collection.
- The Reporting Module shows progress against forecast, retention trends, and other critical indicators, helping teams proactively resolve issues.
Site navigator
Site Navigator provides detailed oversight of site-level activity, highlighting underperforming or blocked locations and reducing delays before first-patient-in.
Data Speak (AI trial intelligence)
Data Speak allows users to ask natural-language questions and receive immediate insights across dashboards and modules, eliminating manual reporting and accelerating decision-making.
Data integration, security and compliance
Axceler8 Rx runs as a single-tenant platform inside each client’s environment, ensuring full data isolation and alignment with internal governance rules. To support the diversity of pharma systems and formats, we built flexible integration flows and adaptable data templates that easily connect to client data sources, from spreadsheets and internal applications to controlled data zones where outbound API calls are restricted.
For security and compliance, Axceler8 Rx keeps production data separate from development, follows strict change-control and validation processes, and includes built-in fallback and rollback mechanisms. This architecture ensures data integrity, traceability, and compliance with the high standards required in global pharma.
The future of clinical trials starts now
Axceler8 Rx has already caught the eye of pharma industry leaders. One of the first adopters is a global, Fortune 500, biopharmaceutical company.
The next step? We now focus on integrating the platform with existing clinical trial management systems to further streamline operations and improve data accessibility.
And we’re just getting started. The future of clinical trial management is here, and we’re leading the way.